Apparatus
New Drug For IDH1 Mutation/Relapsed/Refractory Acute Myeloid Leukaemia With Good Complete Remission Rates And Manageable Toxicity
The results of a new study recently showed that Olutasidenib monotherapy in patients with relapsed/refractory acute myeloid leukaemia carrying the IDH1 mutation elicited high rates of complete remission with manageable toxicity.
Recently, the results of a new study showed that Olutasidenib monotherapy in patients with relapsed/refractory acute myeloid leukemia carrying the IDH1 mutation elicited high rates of complete remission with manageable toxicity.

Olutasidenib (Rezlidhia) monotherapy in patients with relapsed/refractory acute myeloid leukaemia (AML) carrying the IDH1 mutation caused a high complete remission (CR) rate of 32%.
In leukaemia, the three treatment outcomes are usually represented clinically by CR/PR/NR, where CR means complete remission and indicates that the patient is physiologically normal; PR means that some indicators are as normal as normal, but some indicators have not yet reached CR and the outcome is very good; and NR means that current treatment is ineffective. However, even with CR, patients still need to follow up regularly as prescribed by their doctor to avoid relapse.
"Olutasidenib delivered durable remissions with manageable adverse effects," wrote Dr Jorge Cortes of the Augusta University Georgia Cancer Center and colleagues.
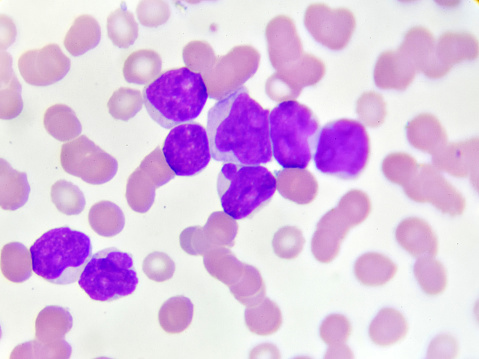
Between 7-14% of AML patients carry the IDH1 mutation. Based on this, inhibiting the IDH1 mutation in tumour cells could help restore normal cell differentiation and provide therapeutic benefit to patients.
Olutasidenib is a potent and selective oral small molecule IDH1 inhibitor that was previously demonstrated in a phase I trial for clinical antitumour activity in patients with IDH1 mutated AML, as well as for safety of tolerability.
The trial enrolled 153 patients with IDH1-mutated, relapsed/refractory AML, all of whom received at least 1 dose of Olutasidenib. The efficacy-assessable population included 147 patients with confirmed CNS diagnosis.
In the Phase II cohort of the open-label, multicentre trial, patients received 150 mg of Olutasidenib twice daily for 28 consecutive days in one cycle.
Of the efficacy assessable population, 50% were male and the mean age was 71.0 years. Overall, 66% had primary new-onset AML and 34% had secondary AML. a total of 35% of patients had refractory disease and 65% had recurrent disease, with an average of 2 treatment visits. In addition, 97% of patients had previously received induction therapy and 12% had received haematopoietic stem cell transplantation (HSCT).
When focusing on patients with CR and CRh (complete remission with partial incomplete haematological recovery), the average duration of remission from treatment was 25.9 months.
In the overall population, the mean overall patient survival was 11.6 months, and for the population of CR+CRh patients in good remission, the estimated 18-month survival rate was 78%.
Regarding safety, 73% of patients experienced various grades of adverse events, with 39% experiencing grade 3 or 4 adverse treatment times. Specifically, the more common treatment adverse events included nausea, leukocytosis, elevated alanine amino transaminase, constipation, fatigue, neutropenia, elevated gamma-glutamyl transferase, and elevated liver enzymes, among others.
Thirty-one per cent of patients experienced emergency events leading to treatment interruption, including disease progression, differentiation syndrome, neutropenic fever and pneumonia.
A total of 48 deaths (31%) were reported in the study, most of which were related to AML progression or complications. The more common adverse events leading to death were disease progression, pneumonia, cerebral haemorrhage, sepsis, pneumonia fungus, respiratory failure and others.
-
![]()
![]() ApparatusDec 24, 2024
ApparatusDec 24, 2024Nature Medicine publishes results of H-drug combined with chemotherapy for first-line treatment of esophageal squamous cell carcinoma
-
![]()
![]() ApparatusDec 23, 2024
ApparatusDec 23, 2024Immune And Astrazeneca Launch Strategic Research Collaboration To Accelerate Drug Target Discovery
-
![]()
![]() ApparatusDec 22, 2024
ApparatusDec 22, 2024The 3D Medical Application Center of the U.S. Army customized assistive devices and artificial limbs for 3D printing of veterans
-
![]()
![]() ApparatusDec 21, 2024
ApparatusDec 21, 2024J. Enzyme Inhib. Med. Chem. | Protac Targeting Aimp2-Dx2: a New Therapy For Lung Cancer
-
![]()
![]() ApparatusDec 20, 2024
ApparatusDec 20, 2024Canada has developed a hand-held detector to help reduce the use of antibiotics