Apparatus
Heavyweight! First ADC Drug For Refractory Ovarian Cancer Receives Accelerated FDA Approval
Elahere, the first antibody-coupled drug (ADC) for refractory ovarian cancer, has received accelerated approval from the FDA, resulting in substantial tumour shrinkage in 31.7% of patients and complete tumour disappearance in 4.8%.

The first antibody-coupled drug (ADC) for refractory ovarian cancer, Elahere, received accelerated FDA approval, resulting in substantial tumour shrinkage in 31.7% of patients and complete tumour disappearance in 4.8% of patients.
Recently, the US FDA granted accelerated approval for Elahere (mirvetuximab soravtansine-gynx) for the treatment of adult patients with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer, requiring these patients to have received 1 to 3 prior lines of systemic therapy.
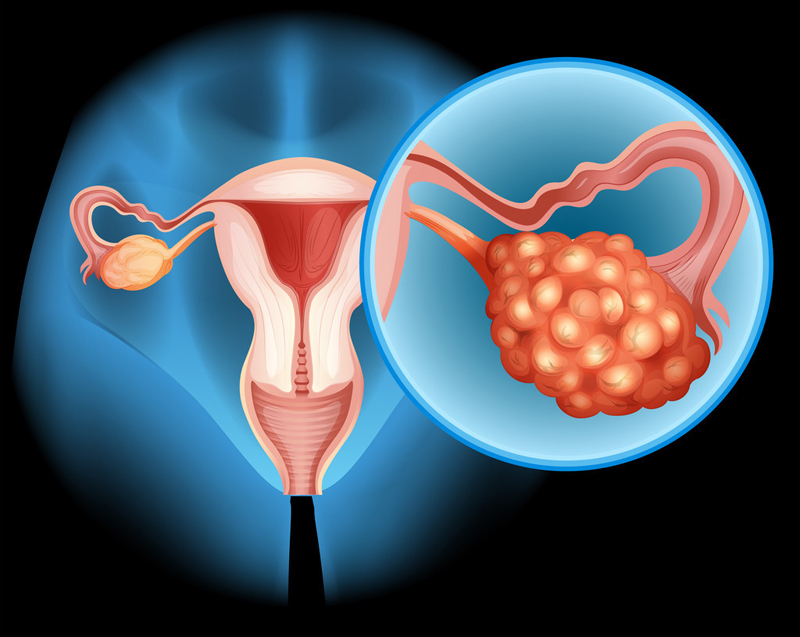
FRα protein is a highly expressed protein in ovarian cancer, with approximately 35% to 40% of ovarian cancer patients expressing high levels of FRα.
Elahere is the first antibody-coupled drug (ADC) to target the FRα protein and the first ADC to be approved for the treatment of platinum-resistant ovarian cancer.
Basis for approval
The effectiveness of Elahere was evaluated in the Phase 3 SORAYA study.
The study enrolled 106 patients with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer. Patients were required to have received up to 3 previous lines of systemic therapy, and all patients were treated with bevacizumab. Patients with ongoing treatment for ocular problems such as corneal disease were not eligible for enrolment.
Enrolled patients were given an intravenous infusion of Elahere at a dose of 6 mg/kg every 3 weeks until disease progression or unacceptable toxicities occurred.
The results showed that 31.7% of patients treated with at least 1 dose of Elahere had a substantial reduction in tumour size, with 4.8% of these patients having complete tumour disappearance, and the efficacy of the treatment lasted for an average of 6.9 months.
Ursula Matulonis, Director of Gynecologic Oncology at the Dana-Farber Cancer Institute and co-principal investigator of the study, said: "The approval of Elahere is significant for patients with FRα-positive, platinum-resistant ovarian cancer, who have limited treatment and a poor prognosis. antitumour activity, durability of efficacy and good tolerability in the trial, indicating that this novel therapy is expected to benefit a large number of patients."

Dr Ursula Matulonis
Safety
In terms of safety, common adverse effects of Elahere treatment include visual impairment, fatigue, elevated liver enzymes, nausea, keratosis, abdominal pain, lymphopenia, peripheral neuropathy, diarrhoea and dry eye.

About ovarian cancer
Ovarian cancer is one of the leading causes of death from gynaecological cancers. Most patients are diagnosed at an advanced stage and usually undergo surgery, and post-operative platinum-based chemotherapy. Unfortunately, most patients will eventually become resistant to platinum-based chemotherapy, making treatment more difficult. In such cases, the effectiveness of subsequent single-agent chemotherapy is very low and the toxicity is high.
The approval of Elahere therefore offers new hope for patients with this type of ovarian cancer.
-
![]()
![]() ApparatusDec 24, 2024
ApparatusDec 24, 2024Nature Medicine publishes results of H-drug combined with chemotherapy for first-line treatment of esophageal squamous cell carcinoma
-
![]()
![]() ApparatusDec 23, 2024
ApparatusDec 23, 2024Immune And Astrazeneca Launch Strategic Research Collaboration To Accelerate Drug Target Discovery
-
![]()
![]() ApparatusDec 22, 2024
ApparatusDec 22, 2024The 3D Medical Application Center of the U.S. Army customized assistive devices and artificial limbs for 3D printing of veterans
-
![]()
![]() ApparatusDec 21, 2024
ApparatusDec 21, 2024J. Enzyme Inhib. Med. Chem. | Protac Targeting Aimp2-Dx2: a New Therapy For Lung Cancer
-
![]()
![]() ApparatusDec 20, 2024
ApparatusDec 20, 2024Canada has developed a hand-held detector to help reduce the use of antibiotics